This service is available in real time upon request with a 30-minute notice 24/7/365.
SANTA CLARA, Calif., August 3, 2022 (Newswire.com) – Zeto, Inc., announced today that it now offers remote routine or continuous electroencephalography (EEG) monitoring services to its customers. At a fixed hourly rate, an accredited monitoring service provides real-time remote video EEG monitoring by registered EEG technologists (R.EEG.T) when such personnel are unavailable on-site. This service is available in real time upon request with a 30-minute notice 24/7/365, at no extra charge during nights, weekends, or holidays.

Driven by the shortage of in-house monitoring capability, this additional service has become increasingly relevant to hospitals and clinics alike to meet the need for timely, high-quality patient care. Zeto is committed to opening its EEG cloud platform for such services as remote work by medical personnel is gaining adoption.
How it works: When Zeto customers need EEG specialists, they simply click on a service request button in the Zeto software, which notifies the monitoring service immediately, and monitoring starts within 30 minutes. By choice, this service can be requested in advance. In real time, an R.EEG.T remotely views the EEG and patient video, making annotations and notifying care providers when needed.
At an affordable hourly flat rate, providers now have the choice to use this simple and quick remote service. Alternatively, providers can use the Zeto Cloud to have their own EEG technologists monitor and annotate from afar. These options are crucial steps that ensure patient needs can be met with minimal delay while complying with standard practices and documentation requirements.
“We believe this service will be beneficial for everyone, including hospitals that would not be limited by the shortage of EEG professionals and can take care of their patients as fast as needed. Patients can have an EEG recorded, monitored, and reviewed for escalation of critical findings within minutes after the doctor orders it, and registered EEG technologists across the U.S. can apply their skills, even if they are not on-site 24/7/365,” said Florian Strelzyk, Chief Sales Officer at Zeto.
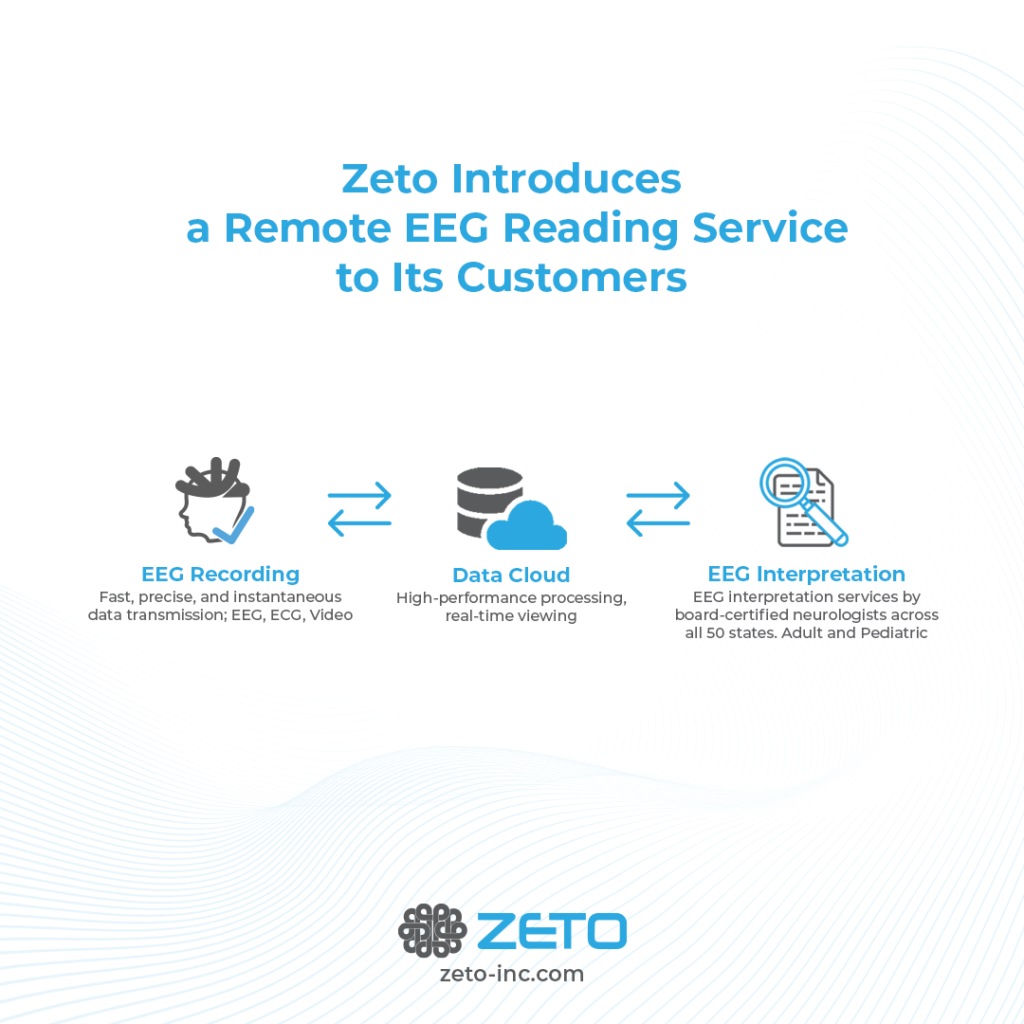
In May, Zeto began offering a remote EEG physician reading service to its customers. This was made possible through a partnership with a physician-led organization dedicated to providing expert, high-quality telemedicine-assisted EEG interpretation to hospitals and outpatient settings, regardless of geographic location.
About Zeto
Zeto, Inc. is an award-winning, privately held medical technology company located in Santa Clara, California, that is focused on transforming the way electroencephalography (EEG) is performed at hospitals and clinics. Zeto’s revolutionary FDA-cleared EEG headset and cloud platform bring the traditional EEG procedure to the 21st century.
To learn more about Zeto’s products, including the remote EEG monitoring, please visit: https://zeto-inc.com/ or email us at info@zetoinc.com.