HUNTSVILLE, TX, June 11, 2025 / PRNewswire/ – Zeto, Inc. is proud to announce the success of a new onsite EEG program at Huntsville Memorial Hospital (HMH) that has helped reduce emergency room patient transfers and improve access to neurological diagnostics for the Huntsville community and surrounding areas. As the only hospital within a 30-mile radius, HMH now offers critical services that previously required transfers to larger facilities in Conroe, The Woodlands, or Houston.
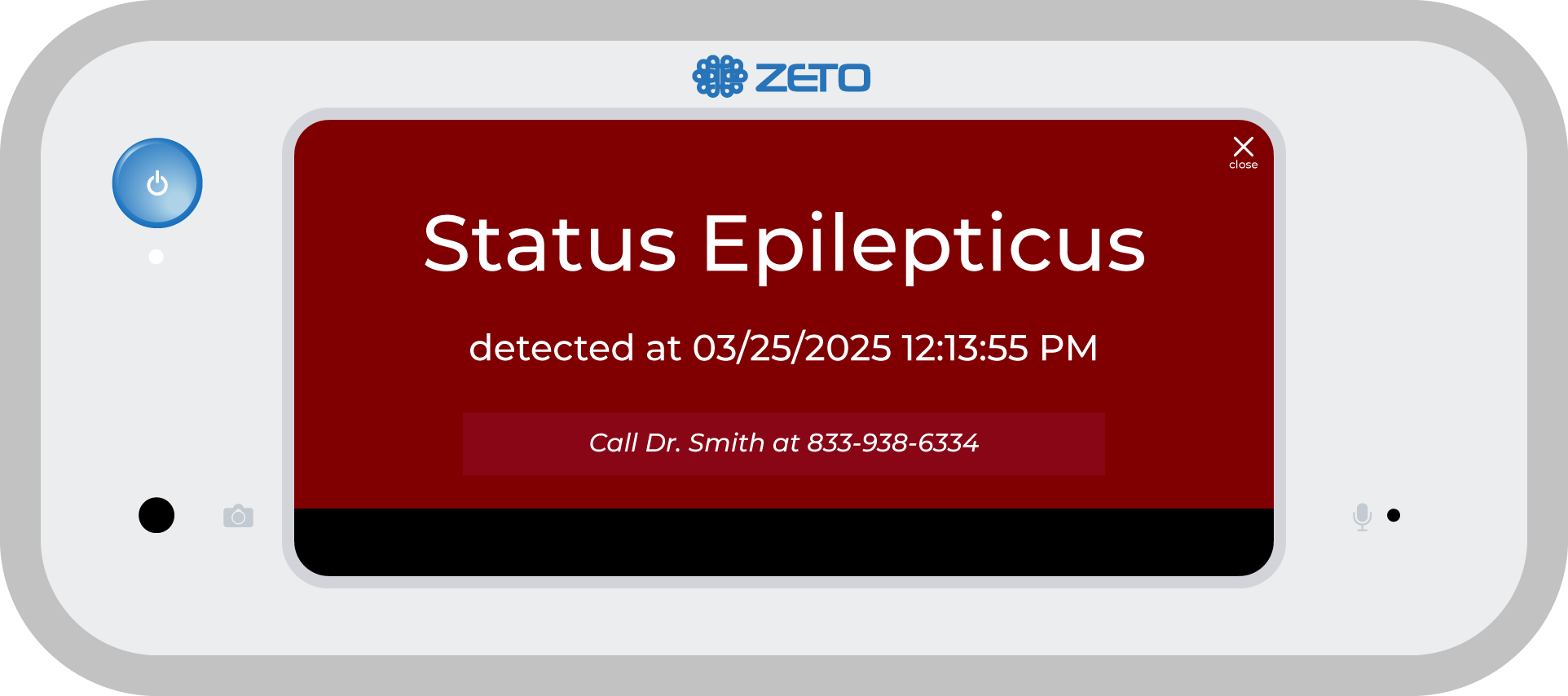
An EEG (electroencephalogram) is a test that measures electrical activity in the brain and is commonly used to diagnose seizures, stroke, and other neurological conditions.
Faced with a high volume of neuro-related transfers and limited access to specialty care, HMH leadership assembled a task force to study ER transfer patterns. “We looked at two years of transfer data and saw that many patients were being transferred for neuro-related issues, particularly when an EEG was needed,” said Joe Schorre, Director of Cardiopulmonary and Emergency Management, who led the EEG implementation at HMH. “Our goal was to turn those transfers into local admissions.”
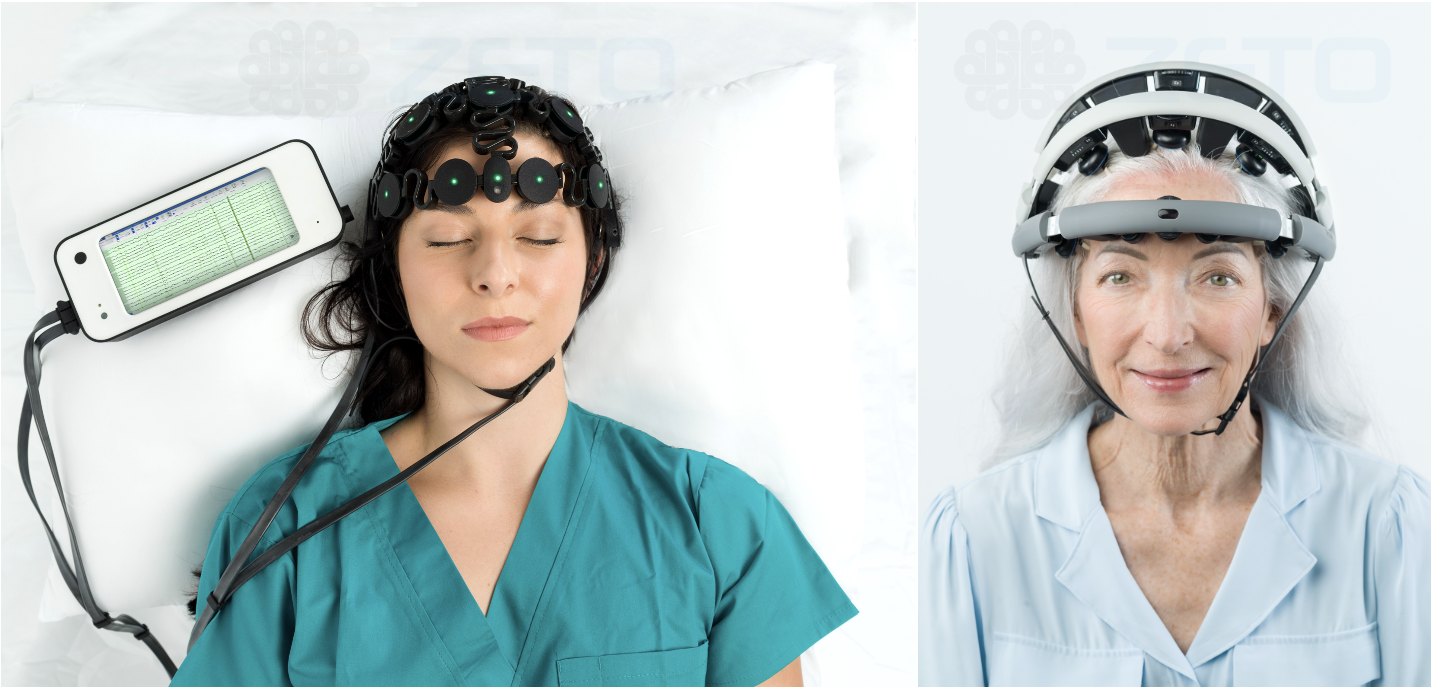
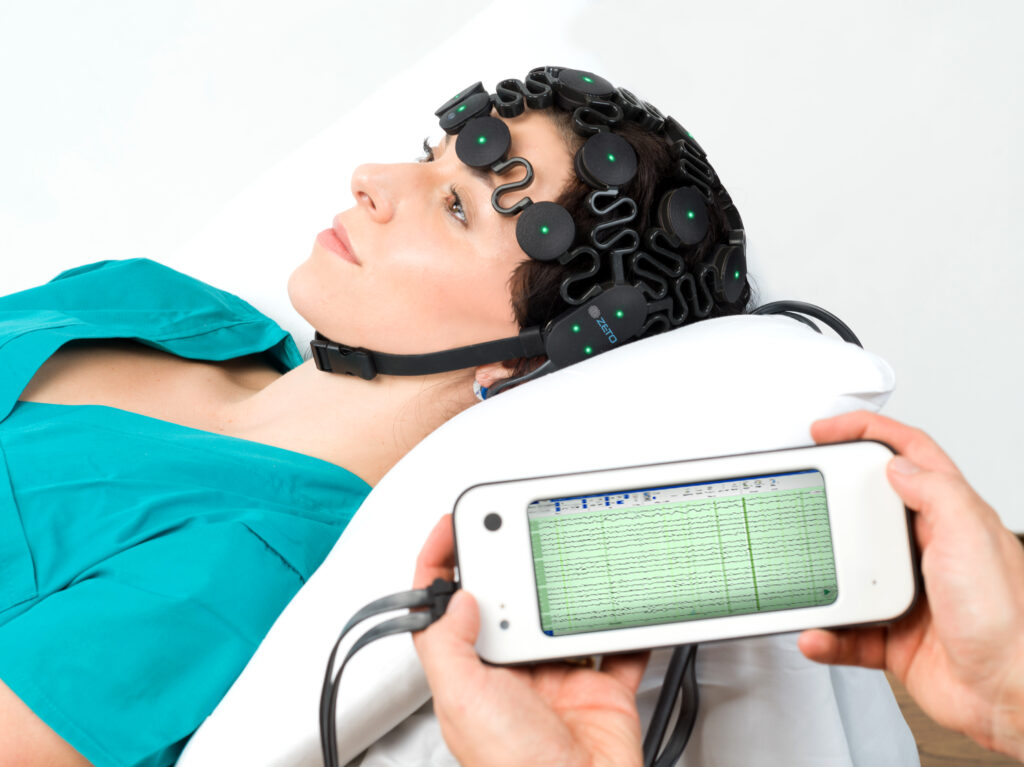
Knowing that the EEG services were needed for Huntsville and Walker County, the hospital explored options for bringing neurological diagnostics in-house as part of a broader inpatient teleneurology and stroke program. The result was the successful implementation of a rapid EEG solution from Zeto that requires no gels or pastes and can be set up by respiratory therapists in under five minutes. “We’re not EEG techs, so we needed a solution that was easy to learn, easy to apply, and could integrate with remote neurologists for interpretation,” said Schorre.
Since the program’s launch in December 2024, HMH has been able to admit more than 40 patients who would otherwise have been transferred to a facility over 30 miles away at a minimum. “Having the ability to implement an EEG program here at HMH is huge step forward for us to elevate the level of care for Walker County residents. Receiving high quality services closer to home which means families and loved ones don’t have to travel, lose time from work, or worry about being separated from loved ones during treatment,” Patrick Shannon, CEO of HMH stated. “For many in our rural community, that makes a huge difference.”
The program has also supported the development of an outpatient EEG program, allowing local primary care providers to refer patients for pre-neurology diagnostic testing. “We’re helping patients get their EEG and MRI here, so by the time they go to the neurologist, they have data in hand,” said Schorre. “It shortens the wait time and gets them the care they need faster.”
Looking ahead, HMH plans to expand services into continuous EEG monitoring and outpatient teleneurology. “This is about doing what’s right for our community. We don’t have local neurology, but we do have smart, committed staff and the tools to support them,” said Schorre.
About Huntsville Memorial Hospital
Huntsville Memorial Hospital, Huntsville, Texas, is currently a 123-bed, CIHQ‐accredited, not‐for‐profit acute care community hospital. HMH delivers quality healthcare services to the residents of Walker County and its surrounding communities, a population of more than 82,000. HMH and its dedicated staff offer compassionate care and the latest technologies and treatment solutions. HMH holds the American Heart Association Primary Stroke Center designation. HMH keeps community health and wellness at the forefront of its mission. Please visit us at www.huntsvillememorial.com.
Source: PRNewswire