The American Heart Association (AHA) recommends prompt electroencephalography (EEG) neuroprognostication for post-cardiac arrest patients in their 2020 guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC). The prompt use of EEG in post-cardiac arrest patients is important because it allows for early identification of brain injury and can guide decisions about the continuation of life-sustaining treatment.
Hypoxic-ischemic brain injury is a leading cause of morbidity and mortality in survivors of hospital cardiac arrest.1 Sadly, most of the post-resuscitation deaths are caused by the active withdrawal of life-sustaining treatment. The decision to actively remove life-sustaining treatment is made when a poor neurological outcome is expected. For this reason, it is critical to perform accurate neuroprognostication, as recommended by the American Heart Association (AHA) in their 2020 guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC).2
Proper post-cardiac arrest neuroprognostication, or EEG after cardiac arrest, is essential to distinguish those who may achieve a meaningful neurological recovery from those who will inevitably have a poor neurological outcome.2,3
Who Should Receive Neuroprognostication After Cardiac Arrest?
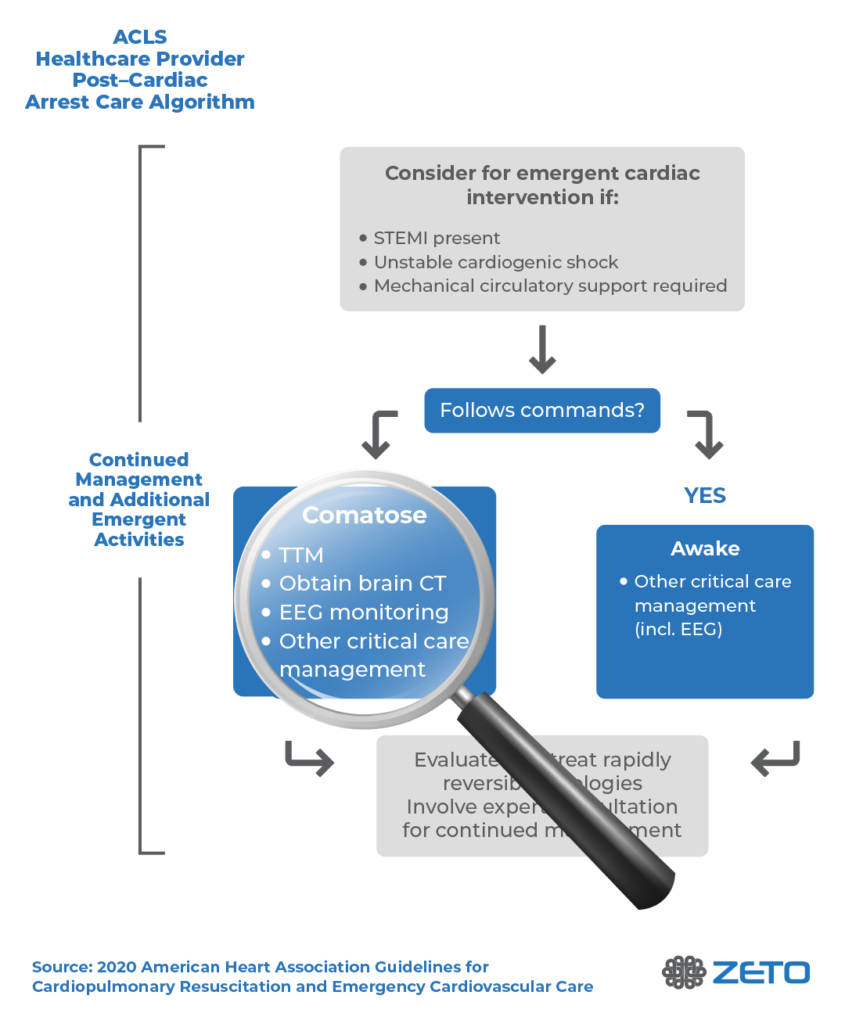
The AHA recommends multimodal neuroprognostication on all patients who remain comatose after cardiac arrest (Level 1 recommendation).2 Multimodal neuroprognostication includes EEG, MRI, quantitative pupillometry, and serum neuron-specific enolase, among others. In addition to neuroprognostication, EEG testing is recommended to identify seizures and, if necessary, provide treatment.
Nonconvulsive seizures, for example, are common after cardiac arrest, and cannot be reliably detected without EEG.4 The American Academy of Neurology also provides data on its importance. Also, EEG after cardiac arrest should be used to rule out underlying ictal activity in cardiac arrest survivors with status myoclonus. The 2020 Emergency Cardiovascular Care Science with Treatment Recommendations (CoSTR) advises seizures to be treated when diagnosed in cardiac arrest patients with return of spontaneous circulation (ROSC).5
When Should Neuroprognostication After Cardiac Arrest Start?
Importantly, prognostic assessments should not be started too early. If they are administered too soon after the cardiac arrest and during initial post-resuscitation care, the results may appear worse than they actually are because of medications, or acute post-injury changes.6 Perhaps surprisingly, clinical prognostic testing such as pupillary light reflex should not be used for neuroprognostication until at least 5 days after ROSC (return of spontaneous circulation) in patients treated with targeted temperature management (TTM), in order for such testing to have prognostic significance.
Testing should not begin until the patient has been normothermic for at least 72 hours.6-8 Imaging or EEG to detect status myoclonus may begin as early as 24 hours after ROSC; two studies including 347 patients, showed the presence of status myoclonus within 72 hours of ROSC predicted poor neurological outcome with specificity of 97% to 100%.9,10 However, postanoxic status epilepticus may not manifest until 72 hours or more after ROSC and sedative drug dosages have been reduced, so waiting for neurological prognosis assessment is necessary.
How to Use EEG for Neuroprognostication After ROSC
The 2020 American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) recommend the use of EEG after cardiac arrest in patients who remain in a coma after ROSC for the purposes of neuroprognostication.2
Findings that are consistent with poor outcomes include postanoxic status epilepticus and/or burst suppression 72 hours or more after ROSC.2 Another potentially useful electrodiagnostic test is the somatosensory evoked potential (SSEP).
SSEP testing is conducted by stimulating the median nerve and looking for a resulting cortical N20 wave. N20 SSEP waves that are absent bilaterally correlate with poor prognosis.2 Likewise, rhythmic periodic discharges on EEG are also consistent with poor prognosis.
Importantly, the AHA notes that the absence of EEG reactivity within 72 hours after cardiac arrest should not be used as the sole determinant of poor neurological outcomes. A lack of EEG reactivity during this time does not necessarily predict a poor neurological outcome.
Obtaining EEG after ROSC
Conventional EEG in the Intensive Care Unit is Critical Care and Cumbersome
While conventional EEG is an acceptable means to obtain EEG in the ICU, it is impractical. Post-cardiac arrest patients who need post-cardiac arrest care in the ICU will, as standard care, be intubated, and have central venous and possibly arterial lines, and intracardiac devices.
Conventional EEG after cardiac arrest is challenging. Attempting to obtain EEG signals through a dozen wires in a critical care setting without quality-limiting artifacts is challenging. Indeed, providing continuous conventional EEG in the ICU is a literal barrier to care for ICU or neurocritical care nurses and staff.
Zeto EEG – Wireless Full-Montage Monitoring For Use on Coma Patients After ROSC

According to the AHA, proper neuroprognostication could prevent withdrawal of life support as it will indicate the accurate neurologic prognosis of a patient who has a chance at successful neurological recovery. Thus, the AHA recommends performing multimodal neurologic prognostication including EEG in all patients who remain in a coma after ROSC following cardiac arrest.
Zeto has developed a full montage, 19-channel (10-20 system) wireless headset with dry electrodes for rapid EEG monitoring. The benefits of Zeto’s electrodes include no skin preparation, no cleanup, and no gel or paste residue. The electrodes are single-use and soft.
The Zeto EEG device collects high-quality EEG recordings and transmits them wirelessly to the cloud for remote viewing and interpretation. ZETO’s EEG platform also offers an FDA-cleared seizure detection and trending algorithm developed by Encevis.
The Zeto EEG headset can be placed after a short training, and the setup takes only 5 minutes – which is crucial for patients in a coma. Once placed, the Zeto headset provides continuous EEG monitoring for up to 5-6 hours.
Overall, prompt EEG for post-cardiac arrest patients is a vital aspect of proper neuroprognostication and plays an important role in determining prognosis in patients and guiding treatment decisions. It is important for healthcare providers, especially intensive care medical providers to be aware of the AHA guidelines and incorporate EEG into their standard care management of post-cardiac arrest patients.
When called upon to perform the difficult and highly consequential task of neuroprognostication, choose Zeto for EEG in the ICU.
References
- Witten L, Gardner R, Holmberg MJ, et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation. 2019;136:93-99. PMID:30710595 doi:10.1016/j.resuscitation.2019.01.031
- Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366-S468. PMID:33081529 doi:10.1161/CIR.0000000000000916
- Geocadin RG, Callaway CW, Fink EL, et al. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation. 2019;140(9):e517-e542. PMID:31291775 doi:10.1161/CIR.0000000000000702
- Freund B, Kaplan PW. Myoclonus After Cardiac Arrest: Where Do We Go From Here? Epilepsy Curr. 2017 Sep-Oct;17(5):265-272. doi: 10.5698/1535-7597.17.5.265. PMID: 29225535; PMCID: PMC5716491.
- Ryoo SM, Jeon SB, Sohn CH, et al. Predicting Outcome With Diffusion-Weighted Imaging in Cardiac Arrest Patients Receiving Hypothermia Therapy: Multicenter Retrospective Cohort Study. Crit Care Med. 2015;43(11):2370-2377. PMID:26284621 doi:10.1097/CCM.0000000000001263
- Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15(1):113-119. PMID:20680517 doi:10.1007/s12028-010-9412-8
- Berg KM, Soar J, Andersen LW, et al. Adult Advanced Life Support: International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation. 2020. PMID:33098922 doi:10.1016/j.resuscitation.2020.09.012
- Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S465-482. PMID:26472996 doi:10.1161/CIR.0000000000000262
- Ruknuddeen MI, Ramadoss R, Rajajee V, Grzeskowiak LE, Rajagopalan RE. Early clinical prediction of neurological outcome following out of hospital cardiac arrest managed with therapeutic hypothermia. Indian J Crit Care Med. 2015;19(6):304-310. PMID:26195855 doi:10.4103/0972-5229.158256
- Zhou SE, Maciel CB, Ormseth CH, Beekman R, Gilmore EJ, Greer DM. Distinct predictive values of current neuroprognostic guidelines in post-cardiac arrest patients. Resuscitation. 2019;139:343-350. PMID:30951843 doi:10.1016/j.resuscitation.2019.03.035